Steel-facing, a form of electroplating, is a procedure in which a microscopically thin layer of pure iron is electrodeposited onto a copper plate. Zinc plates, often used for etching, cannot be steel-faced, although it is possible to copper-face a zinc plate and then steel-face it. Steel facing has many benefits for the printmaker, and there is virtually no loss of detail if the procedure is done correctly. It greatly increases the printing life of a plate. It eliminates the oxidation of color that occurs when hand printing from a copper plate, and it makes plates noticeably easier to wipe because a steel-faced plate holds very little ink in the grain of the surface metal. Steel facing is truly one of the greatest possible tools for refining a color aquatint.
Steel facing takes place in a vertical tank filled with an electrolyte solution made of dissolved metal salts and ions that permit the flow of electricity. The basic idea is to run an electrical current through the solution from a sheet of pure iron to a copper plate, both of which are suspended in the tank. The current causes ions of iron to be deposited onto the face of the copper. Both the copper plate and the iron sheet must be connected to a power source called a rectifier.
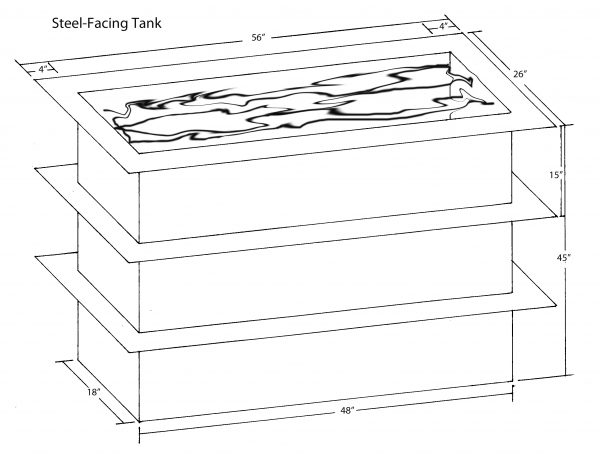
The tank used for steel facing must be watertight, made of a nonconductive material, and large enough to accommodate your largest plate. Crown Point’s tank is fabricated from thick plastic and is a sturdy 45 by 48 by 18 inches. It holds plates up to 36 by 45 inches, the largest size our big press comfortably prints. We have installed a long copper pipe outside the tank, running across its full width and braced about 2 inches above the flat ledge that forms a lip at the tank’s top. We permanently suspend the iron sheet from the pipe, using iron hooks. The positive cable from the rectifier is permanently connected to the copper pipe.
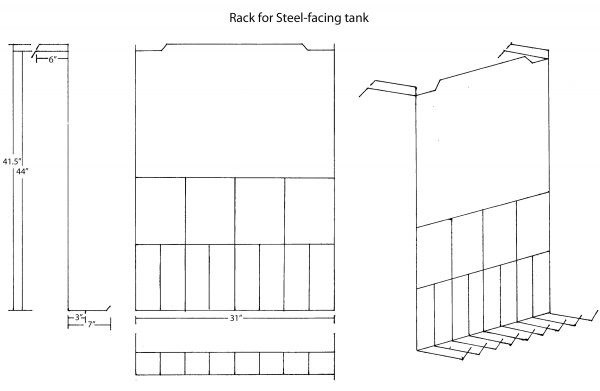
 We have a steel rack fabricated with handles to hang over the interior edge of the tank opposite the edge where the pipe is installed. The rack should not be stored in the tank, as the solution will degrade it. After we have prepared a copper plate for steel facing we place it on the rack and lower it into the tank. We then fasten the negative cable from the rectifier to the steel rack.
We have a steel rack fabricated with handles to hang over the interior edge of the tank opposite the edge where the pipe is installed. The rack should not be stored in the tank, as the solution will degrade it. After we have prepared a copper plate for steel facing we place it on the rack and lower it into the tank. We then fasten the negative cable from the rectifier to the steel rack.
The Electrolyte Solution
1¼ lbs. ammonium chloride to one gallon of distilled water.
The Power Source
At Crown Point, we use a rectifier, which allows us to control the amperage and the voltage of the current. A 25- or 50-amp rectifier that changes to an alternating current to a direct current is ideal.